
Key Takeaways:
- Glucose is our brain’s main fuel source, unless restricted, which leads to ketones becoming the preferred keto fuel source.
- Our brains possess the ability to metabolize glucose and ketones for energy.
- Ketones are metabolized faster than glucose and provide more energy.
Ketones have long been touted as a superior keto fuel source for the brain that possesses a wide array of cognitive benefits. Our brains are made up of two types of cells: neurons and glial cells, and both are imperative for our brains to function properly. [1] Under normal physiological conditions, the principal energy source utilized by the brain cells is glucose. [2] Glucose transporters saturate brain capillaries to allow glucose to cross the blood-brain barrier. Once in the brain, glucose is metabolized to pyruvate, which enters the mitochondria of the brain cells to generate energy through aerobic metabolism. [3]However, ketone bodies may also provide energy to the brain through different mechanisms.
Brain Fuel

In addition to glucose, brain cells can derive energy from monocarboxylates, which include lactate and the ketone bodies beta-hydroxybutyrate (β-HB) and acetoacetate (AcAc). [2] There is debate over whether or not lactate can be used as a keto fuel source in the brain; however, many laboratories have reported that BHB is a major fuel supplier for the brain, especially under specific physiological conditions. [3] [4] BHB and glucose do not nourish the brain uniformly, but rather have specific areas of localization. BHB primarily accumulates in the pituitary and pineal glands, as well as in portions of the hypothalamus and lower cortical layers. Physiological conditions that elevate BHB and consequently provide increased energy to the aforementioned areas of the brain include starvation, fasting, pregnancy, prolonged exercise, uremia during the prenatal period, infancy, the chronic consumption of a high fat/low carbohydrate ketogenic diet, and possibly even ketone supplementation. [4]
Ketones: Brains Utilization Capacity
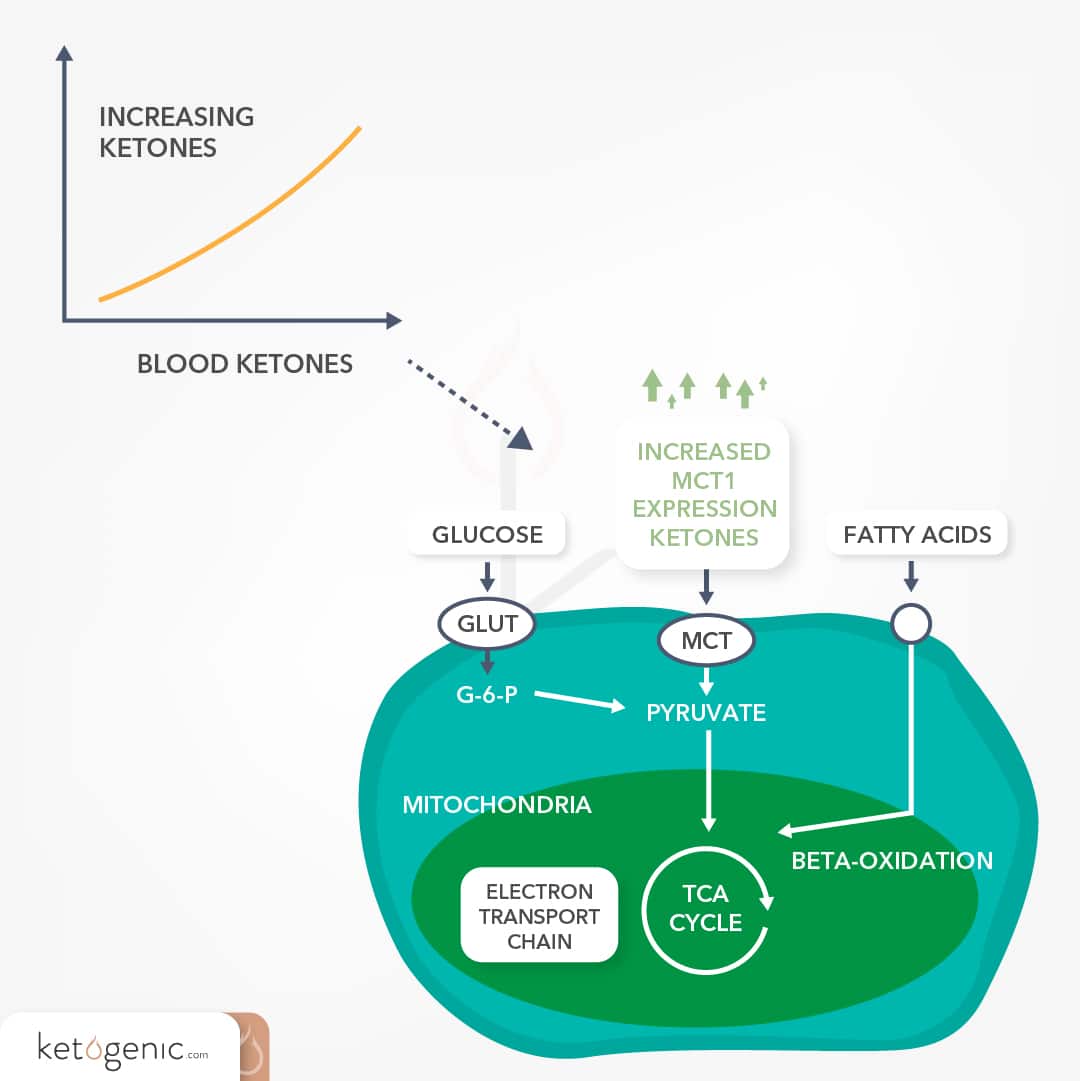
Low circulating glucose levels permit the liver to synthesize ketone bodies using the products that result from the breakdown of fatty acids. Ketone bodies can provide up to 60% of the brain’s energy requirements. This extra fuel source is priceless during times of fasting or starvation, as it maintains brain function and spares vital proteins. [5]Ketone bodies are transported across the blood-brain barrier and into brain cells via monocarboxylate transporters (MCT-1). [2] [3]The rate-limiting step for brain cells to metabolize ketone bodies does not stem from an insufficiency of ketolytic enzymes, but rather from a shortage of MCT-1 expression required for optimal BHB transport into the brain. [4] However, chronic elevations of peripheral circulating ketone bodies enhance MCT-1 expression in the blood-brain barrier [1]; and this increase in MCT-1 expression is followed by a greater brain uptake of BHB and AcAc. [6] Thus, there is an adaptation period at the onset of a ketogenic diet, or starvation, in which elevated circulating ketone levels are required to produce the optimal transport system for enhanced ketone metabolism in the brain. One of the biggest questions is whether or not there is a threshold in which ketones need to be elevated to allow for enhanced transport. Certain research has indicated that the brain’s uptake of ketones is proportional to their production until 12 mmol. [7] [8]
Ketones: Benefits for the Brain

The benefits ensuing from ketone utilization by the brain are multi-faceted. Dr. Krebs [9] revealed the fuel-providing capability of circulating ketone bodies and discovered that their function is analogous to glucose and non-esterified fatty acids. However, ketone bodies may be a preferred keto fuel source for the brain over glucose, due to a greater energetic efficiency. Alternatively, ketone bodies provide more cellular energy per unit of oxygen consumed than glucose. [10] Also, ketone bodies are metabolized faster than glucose; they can bypass the glycolytic pathway and directly enter the citric acid cycle whereas glucose has to first undergo glycolysis. [11] The ketogenic diet is associated with maintaining energy levels which may result from elevated ketone bodies. Ketone bodies modulate leptin and insulin signaling in the hypothalamus to directly affect energy homeostasis and glucose metabolism. [1] Ketone bodies are also a carbon source for glutamate, and therefore balance the glutamate/glutamine ratio in the brain, [12] which may help prevent neurological disorders and conditions. [12] Finally, ketone bodies reduce the production of free radicals, which consequently minimize tissue inflammation. [12] Compared to glucose, ketone bodies are an ideal energy substrate for the brain, as they supply more energy per oxygen consumed, provide energy at a faster rate, regulate energy levels, balance the glutamate/glutamine ratio, and reduce damaging free radicals associated with inflammation.
FAQs About Ketones and the Brain
Does the brain run better on ketones or glucose?
Compared to glucose, ketone bodies are an ideal energy substrate for the brain, as they supply more energy per oxygen consumed, provide energy at a faster rate, regulate energy levels, balance the glutamate/glutamine ratio, and reduce damaging free radicals associated with inflammation.
How does the brain use ketone bodies?
Low circulating glucose levels permit the liver to synthesize ketone bodies using the products that result from the breakdown of fatty acids. Ketone bodies are transported across the blood-brain barrier and into brain cells via monocarboxylate transporters (MCT-1).
References
Seyfried, T. N., & Mukherjee, P. (2005). Targeting energy metabolism in brain cancer: review and hypothesis. Nutrition & metabolism, 2(1), 30.
Hawkins, R. A., & Biebuyck, J. F. (1979). Ketone bodies are selectively used by individual brain regions. Science, 205(4403), 325-327.
Owen, O. E., Morgan, A. P., Kemp, H. G., Sullivan, J. M., Herrera, M. G., & Cahill, G. J. (1967). Brain metabolism during fasting. The Journal of clinical investigation, 46(10), 1589-1595.
Gjedde, A. L. B. E. R. T., & Crone, C. H. R. I. S. T. I. A. N. (1975). Induction processes in blood-brain transfer of ketone bodies during starvation. American Journal of Physiology-Legacy Content, 229(5), 1165-1169.
Krebs, H. A. (1961). The physiological role of the ketone bodies. The third Hopkins Memorial Lecture. Biochemical Journal, 80(2), 225-b2.
Sato, K., Kashiwaya, Y., Keon, C. A., Tsuchiya, N., King, M. T., Radda, G. K., … & Veech, R. L. (1995). Insulin, ketone bodies, and mitochondrial energy transduction. The FASEB Journal, 9(8), 651-658.
Cunnane, S., Nugent, S., Roy, M., Courchesne-Loyer, A., Croteau, E., Tremblay, S., … & Begdouri, H. (2011). Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition, 27(1), 3-20.
Courchesne-Loyer, A., Fortier, M., Tremblay-Mercier, J., Chouinard-Watkins, R., Roy, M., Nugent, S., … & Cunnane, S. C. (2013). Stimulation of mild, sustained ketonemia by medium-chain triacylglycerols in healthy humans: estimated potential contribution to brain energy metabolism. Nutrition, 29(4), 635-640.
Veech, R. L., Chance, B., Kashiwaya, Y., Lardy, H. A., & Cahill, G. F. (2001). Ketone bodies, potential therapeutic uses. IUBMB life, 51(4), 241-247.
LaManna, J. C., Salem, N., Puchowicz, M., Erokwu, B., Koppaka, S., Flask, C., & Lee, Z. (2009). Ketones suppress brain glucose consumption. In Oxygen Transport to Tissue XXX (pp. 301-306). Springer, Boston, MA.
Bak, L. K., Schousboe, A., & Waagepetersen, H. S. (2006). The glutamate/GABA‐glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. Journal of neurochemistry, 98(3), 641-653.









Where are the rest of the references?
Should be there now. Cheers!
References are missing
Should be there now! Enjoy!